vTv THERAPEUTICS
Motivation: Hypoglycemia is a serious medical condition commonly occurring in patients with Type 1 Diabetes (T1D). The risk of severe hypoglycemia [1] increases markedly with the increasing duration of the disease and is a major burden for patients and their families.
Lead Program(s): TTP399 is a novel, oral, small molecule, liver selective glucokinase activator being developed under breakthrough therapy status granted by the FDA as an adjunct therapy to insulin in patients with T1D. TTP399 recently completed a phase 2 study in patients with T1D demonstrating 40% reduction in hypoglycemic events compared to placebo and allowed for tighter long term glycemic control.
Pre-Clinical
Phase 1
Phase 2
Phase 3
Sources:
[1] Frier BM. The incidence and impact of hypoglycemia in type 1 and type 2 diabetes. International Diabetes Monitor 2009;21:210–218

CinCOR Pharma
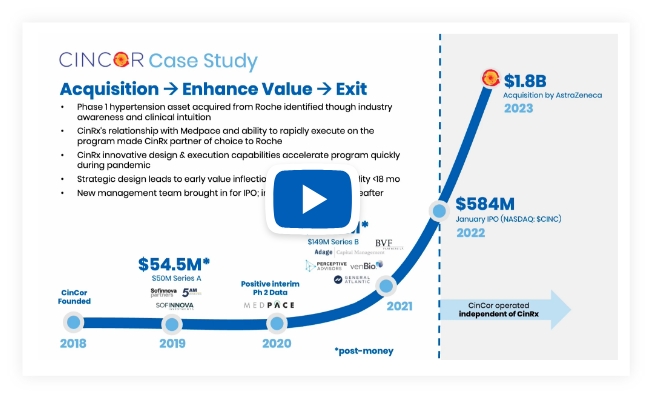
Investment Status: Acquired by AstraZeneca (Jan 2023, $1.8bn)
Motivation: Despite a multitude of approved therapeutics, patients and physicians still struggle to control hypertension – a common but highly dangerous and life-threatening disease.
Lead Program(s): CinCor baxdrostat (CIN-107) is a highly selective, oral small molecule inhibitor of aldosterone synthase currently in phase 2 of clinical development for the treatment of hypertension and other cardio-renal diseases. Baxdrostat has the potential to bring blood pressure into goal range which results in a decreased risk of negative cardiovascular outcomes.
CinDome Pharma
Motivation: 1 in 4 adult patients in the US experience symptoms of gastroparesis – pain, bloating, nausea and vomiting – with the incidence growing rapidly due to the increasing diabetic population [1,2]. A safe and effective treatment that can be taken on a chronic basis remains a significant unmet need.
Lead Program(s): Deudomperidone (CIN-102) is a Dopamine 2/3 antagonist with prokinetic and antiemetic effects being developed to treat patients with gastrointestinal disorders currently in phase 2 of clinical development. Deudomperidone has secured composition of matter patents as well as completed a proof-of-concept study showing signals of improvement in nausea and vomiting and promising effect on gastric emptying in patients with gastroparesis.

Pre-Clinical
Phase 1
Phase 2
Phase 3
Sources:
[1] Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Digestive Diseases and Sciences. 1993;38(9):1569–1580.
[2] Camilleri M, Dubois D, Coulie B, et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clinical Gastroenterology and Hepatology. 2005;3(6):543–552.
CinPhloro Pharma
Motivation: Irritable bowel syndrome (IBS) is one of the most common reasons for a referral to a gastroenterologist at 40%[1]. All currently approved therapies for this chronic and debilitating condition provide sub-optimal treatment for patients and providers presenting a significant unmet medical need.
Lead Program(s): CIN-103 is a modified, pulsatile-release formulation of phloroglucinol designed for sustained symptomatic relief of patients suffering from IBS-D progressing into Phase 2. CIN-103 has secured strong intellectual property for it’s unique formulation and delivery properties.

Pre-Clinical
Phase 1
Phase 2
Phase 3
Sources:
[1] Zaman A. Irritable bowel syndrome. Clin Cornerstone. 2002;4(4):22-33. doi: 10.1016/s1098-3597(02)90003-7. PMID: 12739324.

Cinfina Pharma
Motivation: Obesity is an alarming global public health priority with well-established links to serious diseases including type 2 diabetes, coronary heart disease, stroke, cancer, and chronic obstructive pulmonary disease. While the use of GLP-1s to treat obesity has had dramatic uptake, there is still a tremendous need for more effective therapies with greater weight loss potential.
Lead Program(s): CinFina is developing a series of monotherapy and combination treatments, CIN-109, CIN-110, CIN-209 and CIN-210, currently ranging from pre-clinical to Phase 2. The CinFina portfolio has the potential to transform the treatment of obesity and related cardio-metabolic diseases.

Pre-Clinical
Phase 1
Phase 2
Phase 3
CinSano Pharma
Motivation: Cancer is the #1 cause of death worldwide [1], with solid tumors representing approximately 90% of adult human cancers, and the need for novel therapeutics targeting oncogenes are in demand now more than ever.
Lead Program(s): CIN-108 is a pre-clinical, small molecule that inhibits defective in cullin neddylation 1 (DCN1) from binding in the pocket where it is necessary to promote neddylation. CIN-108 has the potential to significantly interfere with the progression of cancers, including multiple solid tumors.

Pre-Clinical
Phase 1
Phase 2
Phase 3
Sources:
[1] Cancer (who.int)

Retromer Therapeutics
Motivation: Despite multiple attempts to curtail progression of neurodegenerative diseases, few safe & efficacious therapeutics have come to market, underscoring the importance of accelerating novel approaches to a disease modifying therapeutic.
Lead Program(s): Retromer Therapeutics is pioneering a new class of therapeutics to treat neurodegenerative diseases by restoring the function of the endolysosmal trafficking system with preclinical programs in neurodegenerative diseases.
Pre-Clinical
Phase 1
Phase 2
Phase 3